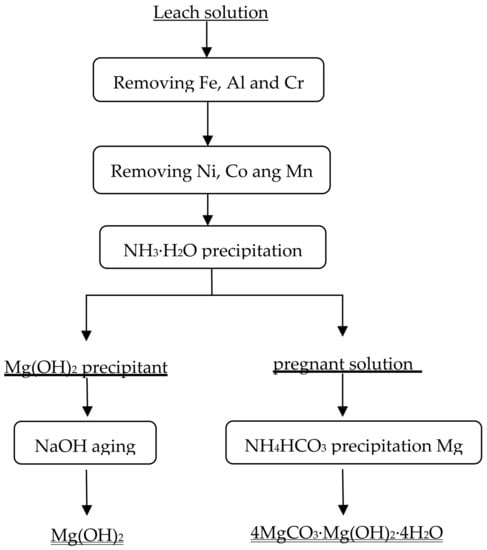
Minerals | Free Full-Text | Recovery of Mg from H2SO4 Leaching Solution of Serpentine to Precipitation of High-Purity Mg(OH)2 and 4MgCO3·Mg(OH)2·4H2O
![SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM](https://cdn.numerade.com/ask_previews/8be369ab-e2f0-4054-a35e-d3e0c372f22f_large.jpg)
SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM

Ni+H2SO4=H2+Ni2(SO4)3 Balanced Equation||Nickel+Sulphuric acid=Hydrogen+Nickel sulphate Balanced Equ - YouTube

a LSVs for the HER on the bare Ni foam (a) and NiBTC/Ni foam (b) in... | Download Scientific Diagram

a HER polarization curves in 1 M H2SO4 for chalcogenide gel on Ni foam,... | Download Scientific Diagram

A Little Nickel Goes a Long Way: Ni Incorporation into Rh2P for Stable Bifunctional Electrocatalytic Water Splitting in Acidic Media | ACS Materials Au

Green separation and recovery of cobalt and nickel from sulphuric acid achieved by complexation-assisted solvent extraction - ScienceDirect

Comparison between experimental and calculated I-E curves. Cu-Ni in 0.5 M H2SO4 + 10 −4 M cysteine: cathodic scan (cf. Fig. 2).

Leaching Kinetics of Mo, Ni, and Al Oxides from Spent Nickel–Molybdenum Hydrodesulfurization Catalyst in H2SO4 Solution | SpringerLink
Effect of H2SO4 concentration on Ni leaching. Conditions: (a) 6 vol.%... | Download Scientific Diagram